Research
Overview
Chromatin, the assemblage of protein, DNA and RNA that represents the physiologic form of the eukaryotic genome, imposes a million-fold length-scale compaction to fit DNA in the nucleus. Rather than serving as mere static packaging, chromatin structure acts as a dynamic regulator of underlying DNA function. Apart from DNA sequence-specific transcription factors, the information carriers responsible for this structural variation are chemical modifications to the genome itself and attendant histone proteins involved in packaging (often referred to as “epigenetic” marks), as well as noncoding RNA acting at the chromatin interface. Although little known about these epigenetic information carriers, it is clear that they play crucial roles in development, cognition and disease. The unifying theme of the Ruthenburg lab is the elucidation of the molecular mechanisms by which epigenetic information carriers impact chromatin structure and function, particularly in the context of transcriptional activation.
The Ruthenburg lab research program spans a host of traditional disciplines (discovery biochemistry, chemical biology, biophysics, technology development, quantitative genomics and cell biology) with the goal of developing fundamental mechanistic understanding of epigenetic information systems through three main avenues.
1. Accurate measurement of the individual marks and patterns is a crucial prerequisite to deciphering how epigenetic systems work. To address this and other substantial problems with the field’s central tool, we developed internally calibrated chromatin immunoprecipitation (ICeChIP), which uses barcoded semisynthetic nucleosomal calibrants to enable absolute quantification of histone marks and variants. This method quantitatively probes finer scale chromatin features in cells than previously possible, enabling new discoveries and resolving longstanding questions in the field.
2. We have discovered a new class of long noncoding RNA molecules, which display potent enhancer activity and are defined by tight attachment to their site of transcription.We have developed compelling evidence for their generality and begun defining their molecular mechanisms.
3. A third major effort in the lab is the biochemical discovery of new epigenetic pathways, centered on specific binding partners for orphaned histone marks and DNA-modifications.These fundamental problems animate our inquiry into the molecular underpinnings of epigenetic information flow, driven by new quantitative approaches coupled with rigorous biochemistry as detailed below.
Advances in quantitative interrogation of chromatin states
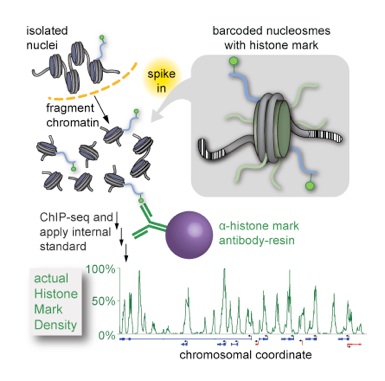
The fundamental repeating unit of chromatin structure is the nucleosome—a spool of eight histone proteins, around which nearly two full turns of DNA are wrapped. Post-translational modifications on the histone constituents of nucleosomes are able to transduce changes in local chromatin states that govern the accessibility of underlying DNA, regulating processes that range from transcriptional activation to gene silencing.
Despite serving as the central experimental technique in epigenetics research for mapping these marks, conventional chromatin immunoprecipitation coupled to deep sequencing (ChIP-seq) suffers from serious drawbacks: it is a relative measurement untethered to any external scale in a way that obviates direct comparison amongst experiments and it employs antibody reagents that have differing specificities and affinities for epitopes, which are in turn variable in abundance. Yet none of these factors are considered in conventional analysis. To address these substantial problems and probe a new layer of chromatin variation, we devised ICeChIP, a method that “spikes in” a series of barcoded semisynthetic nucleosomes as internal standards to calibrate ChIP, so that:
1. data is expressed on an precise, accurate, reproducible, and biologically meaningful scale of histone modification density, rather than an arbitrary and relative scale that is restricted to experimental reference frame;
2. this calibrated scale, when applied from experiment to experiment, enables unbiased quantitative comparisons between experiments; and
3. quantification of IP specificity for the calibrant serves as a critical in situ metric of whether the experiment actually worked (Grzybowski et al., Mol. Cell 2015). We feel that ICeChIP is transformative—it has already revealed paradigm-shifting insight into the nature of histone marks and variant patterns on the nucleosomal scale, as detailed below.
In stem and progenitor cells, the chromatin states demarcating genes that are poised to be rapidly activated or fully repressed along a potential differentiation axis are thought to bear the apparent juxtaposition of transcriptionally activating and repressive histone methyllysine marks, H3K4me3 and H3K27me3, respectively, termed a “bivalent domain”. Despite a decade of intensive study, we lack a precise molecular description of what constitutes bivalency since ChIP and sequential ChIP measurements designed to probe the nature of apparent mark coexistence fall short of unambiguous molecular detail.

Using ICeChIP measurements of the amounts of both H3K4me3 and H3K27me3 in mESCs, we initially made a conservative statistical argument for the existence of bivalency’s bona fide existence at ~35% of all genes in mouse embryonic stem cells (mESCs, Grzybowski et al., Mol. Cell 2015). Our more recent direct measurements indicate this number is closer to 66% (Grzybowski and Shah et al., in revision), far more common than expected! Our measurements of the amounts of these two marks at promoters suggest that bivalency is divided into two classes with reciprocal mark ascendancy: canonical early developmental genes with very high repressive H3K27me3 and modest H3K4me3 as previously described, and genes with promoter H3K4me3 approaching 100% while bearing significant amounts of H3K27me3, including highly active housekeeping genes. In previous experiments, this latter class of genes was entirely missed due to technical flaws in conventional ChIP normalization.
Previous activity assays of the histone methyltransferase complexes that install these marks suggested antagonism by opposing marks bivalent nucleosomes that might preclude their ubiquity in rapidly cycling ES cells. However, in our more systematic approach, we find no evidence that pre-existing H3K27me3 inhibits any of the MLL/Set1 core complexes on nucleosomes, providing a plausible path for the biogenesis of bivalent nucleosomes at the levels we observe by ICeChIP.
We have also used ICeChIP to define new and unexpected quantitative relationships between histone modifications at enhancers and the expression level of their target genes (Shah et al., Mol Cell 2018). In collaboration with Jerome Jullien and John Gurdon, we used ICeChIP to find the first evidence of trans-generational epigenetic inheritance of histone modifications (Oikawa et al., manuscript submitted). In other collaborative efforts, we have deployed ICeChIP to quantify histone marks to define an acute myeloid leukemia subclass that is susceptible to a new rational combination therapy in mouse PDX models (Maganti et al., Cancer Discovery 2018), and how certain VDJ recombination events are driven by ablation of a histone repressive mark (Karki et al., Cell Rep 2018). We have also developed efficient semi-syntheses of the reagents needed to more rigorously probe important histone marks (Chen et al., ChemBioChem 2014), and conclusively demonstrated the deficiencies of alternative approaches to histone methyl lysine mimics via a combination of in-house structural biology, binding biophysics, and nucleosomal affinity measurements (Chen et al., Biochemistry 2018). Moreover, we have played crucial roles in ongoing antibody characterization (Rothbart et al., Mol. Cell 2015) and development (Hattori et al., Nat Meth 2013; Hattori et al., Proc Nat Acad Sci 2016). In our own work, we have found that many widely-used antibodies and literature paradigms are flawed, while discovering viable alternatives (Shah et al., Mol Cell 2018).
CheRNA: a new class of tissue-specific long noncoding RNAs
Distal enhancers have emerged as key regulators of staged gene activation during development. Despite an expanding set of mechanisms by which enhancers activate transcription, the detailed regulatory principles remain elusive. Although progress has been made in predicting active enhancers using stereotyped chromatin mark signatures and the production of short bidirectional transcripts from enhancers themselves (eRNA) as indicators, robust enhancer annotation from genome-scale experiments remains a major challenge. Several noncoding RNAs (ncRNA) including eRNA have been demonstrated to promote transcriptional activation of coding genes at a distance by strengthening of promoter-enhancer looping (through stabilization of bridging protein factors), and/or recruiting histone modification complexes. In light of the recently appreciated promiscuity of ncRNA-protein interfaces (and our own similar results in extensive quantitative examination of ncRNA-interfaces to the MLL1 complex and constituent subunits), how ncRNA interactions can achieve any of their implicated roles with such limited specificity has become a central question. One possible resolution to this conundrum could be immobilization and action of some ncRNAs at their site of production.
We discovered a new class of long ncRNAs biochemically defined by tight chromatin-engagement as a consequence of their ongoing transcription (Werner and Ruthenburg, Cell Rep 2015). Several thousand of these chromatin enriched RNA (cheRNA) species were previously undetected due to their low copy number per cell. These cheRNA are molecularly distinct from eRNA (only ~10% in common), resembling canonical long noncoding RNAs in their unidirectional transcription, length and other properties. Remarkably, cheRNA exhibit stronger correlation to proximal gene expression than eRNA, long ncRNA, or strong enhancers annotated based on chromatin signatures. We envision that our biochemical approach to chromatin-attached ncRNA enrichment will become more widespread in its application, as it offers the best prediction of cis-gene activity available.
More recently, we have developed compelling evidence for the generality of cheRNA-- this cis-gene activation correlation extends to every cell line we have examined. However, they are almost exclusively cell type- and target gene-specific in their expression patterns, consistent with the hypothesis that they play a role in specifying cellular identity (Werner et al., Nat Struct Mol Biol 2017) Targeted depletion of several candidate cheRNA by CRISPRi and/or antisense oligonucleotides causes proportional decreases in neighboring gene expression, establishing a direct role for cheRNA molecules in gene activation. In collaboration with the Moskowitz lab, we have found similar molecules contribute to cardiac development and conduction driven by the TBX5 transcription factor (Yang et al., eLife 2017). As the protein factors reported by others to mediate cis-acting ncRNAs do not significantly impact several candidate cheRNA activities, we are actively exploring the mechanism(s) by which cheRNA potentiate neighboring gene transcription, and have developed evidence for chromosomal architectural effects in several cases.
Biochemical discovery of new DNA modification epigenetics
This work is ongoing, in revision for Molecular Cell (available on BioRxiv 511394).
For 5-methylcytosine (5mC), the paradigmatic epigenetic information carrier, clear mechanisms by which it represses local transcription have long been established. Yet other enzymatically installed DNA-modifications have not been subjected to similar scrutiny as signaling molecules. The discovery that the TET family of enzymes can catalyze sequential oxidation of 5mC to 5-hydroxymethylcytosine (5hmC) then to higher oxidation forms that are subject to reversion to unmodified C, raises the possibility that the epigenetic language of covalent modifications to the 5-position of cytosine may be far more elaborate. While low in abundance in many tissues, 5hmC accumulates to ~0.7-1.5% of all C in embryonic stem cells and cortical neurons, particularly enriched within lineage-specific genes and enhancers, and persists with a half-life comparable to that of its more abundant 5mC precursor. Thus, these newly appreciated oxidized methyl species in DNA (collectively referred to as 5[ox]mC) may not be merely intermediates in the remediation of mC to C. Rather, they may also function as site-specific signaling molecules in the genome distinct from mC. As the TET enzymes play important roles in development, fertility, synaptic plasticity and cancer, discerning whether these effects are exerted by loss of mC-based repression, or by orthogonal signaling pathways initiated by specific-5[ox]mC binding factors remains a crucial mechanistic question.
We posit specific chromatin signaling roles for these oxidative adducts of 5mC by analogy to the well-established epigenetic paradigm of 5mC-- an information carrying moiety embedded in DNA coupled to cognate binding partners that translate the mark into local signaling output. Although this hypothesis is not new, evidence for such signaling pathways, in the form of validated binding factors with appreciable discrimination for particular 5[ox]mC species coupled to important biological function, has been lacking. Given the scarcity of 5[ox]mC modifications compared to their far more abundant precursors, binding selectivity sufficient to overcome this disparity is a minimal requirement for distinct genomic localization to be imparted by such a binding event.
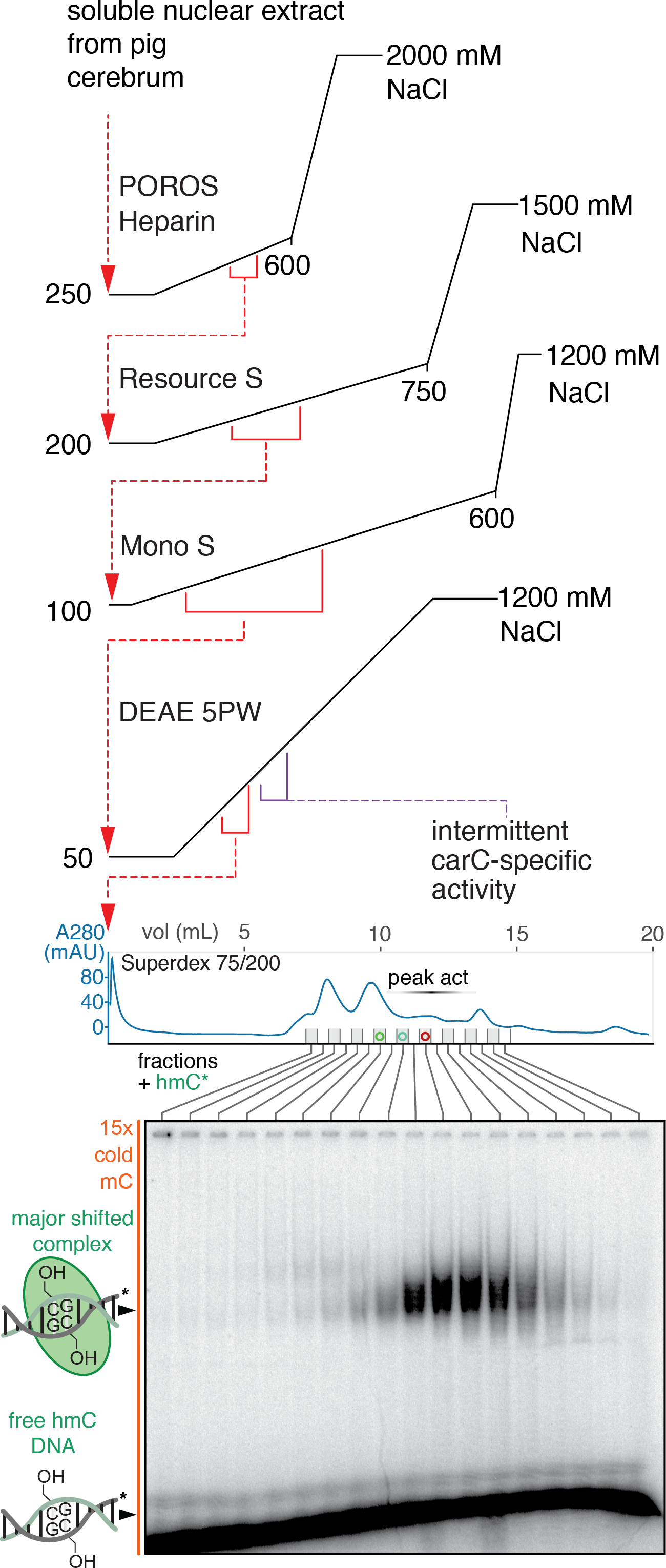
Our lab has found highly-specific binding partners for each of the 5[ox]mC species in the context of duplex DNA by biochemical fractionation of pig brain nuclear extracts and has demonstrated that these molecules are capable of functionally important chromatin signaling. We have focused intensively on validating and exploring the biology of one specific 5hmC-binding partner, WDR76. The purified recombinant human homolog of WDR76 binds directly to 5hmC with sufficient specificity to enable distinct genomic engagement of this low abundance modification. In mESCs, a number of genes and neighborhoods that are 5hmC-rich display Wdr76 binding and display significantly reduced expression upon knockout of Wdr76, consistent with the protein potentiating gene expression as a function of 5hmC binding. While this effect only accounts for a small fraction of 5hmC in mESCs, seemingly restricted to a specific sequence motif of 5hmC display, we are able to demonstrate direct activity of WDR76 in the expression of these target genes. Intriguingly, WDR76 is overexpressed in a class of human leukemias that are dependent on active TET1 enzyme that installs 5hmC. In collaboration with Jianjun Chen (City of Hope), we have found that Wdr76 is oncogenic in MLL1-rearranged leukemia mouse models, phenocopying the gain- and loss-of-function of the notionally upstream TET1 enzyme. Wdr76 in combination with rearranged MLL1 accelerates engrafted mouse mortality by nearly a factor of two relative to rearranged MLL1 alone, yet a point mutant of Wdr76 that is hmC-DNA-binding deficient does not. We anticipate that this study will be the exemplar of several future 5[ox]mC-binding partner papers from our lab that capitalize on our initial biochemical discoveries to vastly expand the field of DNA modification-based epigenetics.
